Pressure-dependent kinetics of the o-xylene reaction with OH radicals†
Abstract
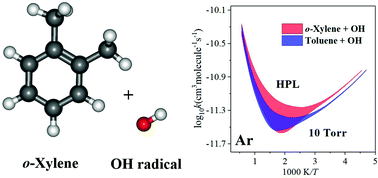
OH-initiated oxidation reactions of o-xylene are widely concerned both in combustion and atmospheric chemistry. In this work, the kinetics of the o-xylene reaction with OH radicals has been studied systematically in a wide temperature range of 220–3000 K for the high-pressure limit and several selected pressures from 1 torr to 500 atm using multi-structural variational transition state theory with the small-curvature tunneling approximation (MS-CVT/SCT) and the system-specific quantum Rice–Ramsperger–Kassel (SS-QRRK) method. The calculations fully considered various factors which could affect the accuracy of the calculated rate constants including anharmonicity of both low- and high-frequency modes and multiple low-energy conformers, variational effect, and tunneling. The results are in good agreement with the available experimental data. The obtained overall rate constants exhibit a nonmonotonic temperature dependence due to the competition between the hydrogen abstraction and addition reactions. At low temperatures, the addition channels are dominant reactions, but the abstraction reactions are also non-ignorable with a ∼12% contribution to the overall rate constants at 298 K and 1 atm. Above 800 K, the abstraction reactions become dominant under all the pressure conditions. In addition, we observed a more significant pressure dependence of o-xylene plus OH reaction as compared to the similar toluene plus OH reaction, which is the effect of the additional methyl group. At T = 500–1000 K, the pressure can influence the total rate constants of the o-xylene reaction by a factor of up to 2.5. These kinetics data provide us with a comprehensive understanding of the mechanism and pressure-dependence of kinetics for the o-xylene + OH reaction, which is also beneficial for the study of other similar aromatic hydrocarbon reactions.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...