Thermodynamics of hydrogels for applications in atmospheric water harvesting, evaporation, and desalination†
Abstract
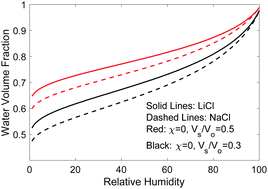
Most thermodynamic modeling of hydrogels is built on Flory's theories for the entropy of mixing and rubber elasticity, and Donnan's equilibrium conditions if polyelectrolyte polymer and mobile ions are involved. The entropy of mixing depends on the number of solvent and polymer molecules while the configurational entropy depends on the volume the polymer occupied. Flory's theory treated these two entropy terms in the Gibbs free energy on an equal basis: using the molecular numbers as the variable. I argue that the molecular number and volume are two independent thermodynamic variables and reformulate Flory's classical hydrogel thermodynamic model by minimizing the Helmholtz free energy of a combined system consisting of the hydrogel and its environment. This treatment enables us to unequivocally state that the osmotic pressure is the thermodynamic pressure of the solvent inside the hydrogel and to unambiguously write down the chemical potential of each species. The balance of the chemical potentials of the mobile species, including both the solvent and the mobile ions gives a set of equations that can be simultaneously used to solve for the equilibrium volume of the hydrogel, the osmotic pressure, and the Donnan potential, including their coupling. The model is used to study the thermodynamic properties of both pure and salty water in non-electrolyte and electrolyte hydrogels such as (1) the latent heat of evaporation, (2) the ability of hydrogels to retain water and to absorb water from the atmosphere, (3) the use of hydrogels for desalination via solar or forward osmosis, (4) the antifouling characteristics of hydrogels, and (5) melting point suppression and boiling point elevation, and solubility of salts in hydrogels. These properties are of interest in solar-driven interfacial water evaporation for desalination and wastewater treatment, atmospheric water harvesting, and forward osmosis. The reformulated thermodynamic framework will also be useful for understanding polymer electrolytes and ion transport in electrochemical and biological systems.


 Please wait while we load your content...
Please wait while we load your content...