Structural and electronic properties of pristine and hydrogen-terminated c-BN(100) surfaces†
Abstract
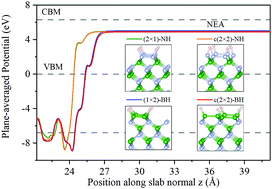
Semiconductor surfaces are crucially important for electronics, but it is difficult to directly image their surface structures. In this work, the surface structures and electronic properties of pristine and H-terminated c-BN(100) surfaces are predicted by first principles calculation. It is found that the surfaces with reconstructed dimers and staggered dimers are thermally and dynamically stable. When the surface N and B atoms are saturated with the virtual H of 0.5 e and 1.5 e, the surface states near Fermi level are nearly removed, and have the wide bandgaps. Meanwhile, after surface hydrogenation, the electron affinity value changes from positive to negative. Our findings could provide a theoretical guidance for designing c-BN-based electronic devices.


 Please wait while we load your content...
Please wait while we load your content...