Inclusion complexes of the macrocycle nonactin with benchmark protonated amines: aniline and serine†
Abstract
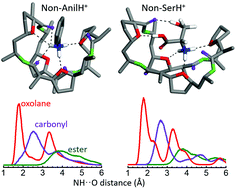
The biological activity of the macrocycle nonactin is intimately related to its ionophore properties and ability to act as a selective cation carrier. While the focus of most investigations on nonactin has been on the binding of metal cations and small molecular ions, this study pursues the characterization of its inclusion complexes with primary amines with bulky structured side groups of different polarity. To this end, the complexes of nonactin with aniline and with the amino acid L-serine, both in protonated form, are considered as case studies and their relevant coordination arrangements are assessed by means of infrared action spectroscopy, quantum chemical density functional theory and Born–Oppenheimer molecular dynamics. The study suggests that the oxygen atoms from the oxolane (tetrahydrofuran) groups of nonactin constitute the preferential docking sites of the ammonium moiety of the guest cation, although conformational constraints promote interactions with the ester carbonyl backbone groups. In the aniline complex, the benzyl side ring is oriented outwards from the cavity, whereas in the case of L-serine, the side carboxylic acid and alcohol groups participate actively in the coordination process. Interestingly, the accommodation of L-serine is favoured when nonactin adopts an enantiomeric-selective folding, that promotes the tripodal coordination of the protonated amine group with oxolane rings from three nonactinic acid blocks with enantiomeric sequence (+)-(−)-(+), which allows for a facile coordination of the serine side groups. This is recognized as a general feature associated with the alternation of chiral domains in globally achiral natural nonactin, yielding mirror-symmetric complexes with the enantiomers of chiral amines.


 Please wait while we load your content...
Please wait while we load your content...