A detailed assessment on the interaction of sodium alginate with a surface-active ionic liquid and a conventional surfactant: a multitechnique approach†
Abstract
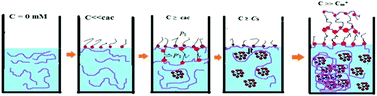
An investigation has been made on the interaction of a biodegradable anionic polyelectrolyte, sodium alginate (NaAlg), with two oppositely charged cationic surfactants, 1-hexadecyl-3-methyl imidazolium chloride (C16MImCl) and 1-hexadecyl triphenylphosphonium bromide (C16TPB), the former is a surface active ionic liquid (SAIL) and the latter a conventional surfactant over a wide concentration regime of the polyelectrolyte. Dual influence of electrostatic and hydrophobic interactions plays in this investigation when mixing surfactants with an oppositely charged polyelectrolyte. A number of different experimental techniques, e.g., conductometry, tensiometry, steady state and time resolved spectrofluorimetry, turbidimetry, isothermal titration calorimetry (ITC), dynamic light scattering (DLS), attenuated total reflection (ATR), high resolution transmission electron microscopy (HR-TEM) and fluorescence microscopy, have been implemented to get comprehensive information about the interaction of the oppositely charged polyelectrolyte and surfactants. Tensiometry study reveals the existence of several conformations of NaAlg influenced by different concentrations of surfactants titrated to it and these are abbreviated as critical aggregation concentration (cac), saturated concentration of polymer-surfactant complex (Cs) and finally extended critical micelle concentration 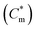 due to the aggregation of the surfactant itself, appearing in chronological order from low to high concentrations of surfactants. Apart from tensiometry, these above concentrations have been well found and the values are well comparable when investigating polyelectrolyte-surfactant interaction by other physicochemical techniques as well. Irreversible phase separation of the oppositely charged polyelectrolyte-surfactant complex (PS-complex) occurs at higher polyelectrolyte concentration investigated here for both the surfactants in the vicinity of cac for C16MImCl and near
due to the aggregation of the surfactant itself, appearing in chronological order from low to high concentrations of surfactants. Apart from tensiometry, these above concentrations have been well found and the values are well comparable when investigating polyelectrolyte-surfactant interaction by other physicochemical techniques as well. Irreversible phase separation of the oppositely charged polyelectrolyte-surfactant complex (PS-complex) occurs at higher polyelectrolyte concentration investigated here for both the surfactants in the vicinity of cac for C16MImCl and near 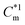 for C16TPB and finally persists after further addition of surfactants above the formation of free micelles. Several bulk and interfacial parameters, viz., Gibbs free energy of micellization
for C16TPB and finally persists after further addition of surfactants above the formation of free micelles. Several bulk and interfacial parameters, viz., Gibbs free energy of micellization 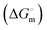 , enthalpy of micellization
, enthalpy of micellization 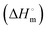 , entropy of micellization
, entropy of micellization 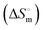 , degree of counterion binding (β), surface excess at cmc (Γmax), area minimum (Amin), surface pressure at cmc (πcmc), pC20, packing parameter (P), hydrodynamic radius (r) and aggregation number (Na) of two surfactants both in the presence and absence of NaAlg, have been calculated for these investigated systems. Characterization of NaAlg, both surfactants and their individual complexes was performed using FTIR-ATR. DLS shows the distribution of size of polymer surfactant complexes over a wide range of surfactant concentrations at a fixed polyelectrolyte concentration, while HR-TEM study reveals not only the size of agglomerated clusters of the PS-complex but also its shapes. Images of NaAlg-surfactant complexes were also captured using fluorescence microscopy in solution phase. A strong PS-complex in the presence of C16MImCl has been reported here over C16TPB.
, degree of counterion binding (β), surface excess at cmc (Γmax), area minimum (Amin), surface pressure at cmc (πcmc), pC20, packing parameter (P), hydrodynamic radius (r) and aggregation number (Na) of two surfactants both in the presence and absence of NaAlg, have been calculated for these investigated systems. Characterization of NaAlg, both surfactants and their individual complexes was performed using FTIR-ATR. DLS shows the distribution of size of polymer surfactant complexes over a wide range of surfactant concentrations at a fixed polyelectrolyte concentration, while HR-TEM study reveals not only the size of agglomerated clusters of the PS-complex but also its shapes. Images of NaAlg-surfactant complexes were also captured using fluorescence microscopy in solution phase. A strong PS-complex in the presence of C16MImCl has been reported here over C16TPB.


 Please wait while we load your content...
Please wait while we load your content...