Molecular dynamic simulation of prenucleation of apatite at a type I collagen template: ion association and mineralization control†
Abstract
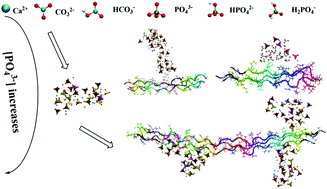
Biomineralization is a vital physiological process in living organisms, hence elucidating its mechanism is crucial in the optimization of controllable biomaterial preparation with hydroxyapatite and collagen, which could provide information for the design of innovative biomaterials. However, the mechanisms by which minerals and collagen interact in various ionic environments are unclear. Here, we applied molecular dynamics and free energy simulations to clarify type I collagen-mediated HAP prenucleation and simulated the physiological environment using different phosphate and carbonate protonation states. Calcium phosphate mineral formation on the type I collagen surface drastically differed among various H2PO4−, HPO42−, PO43−, CO32−, and HCO3− compositions. Our simulations indicated that the presence of HPO42− in the solution phase is critical to regulate the apatite nucleation, whereas the presence of H2PO4− may be inhibitory. The inclusion of CO32− in the solution might promote calcium phosphate cluster formation. In contrast, apatite cluster size may be regulated by changing the anion concentration ratios, including PO43−/HPO42− and PO43−/CO32−. Our free energy simulations attributed these phenomena to relative differences in binding thermostability and ion association kinetics. Our simulations provide a theoretical approach toward the effective control of collagen mineralization and the preparation of novel biomaterials.


 Please wait while we load your content...
Please wait while we load your content...