The mechanism and impact of mono/bis(iodoimidazolium) halogen bond donor catalysts on Michael addition of indole with trans-crotonophenone: DFT calculations†
Abstract
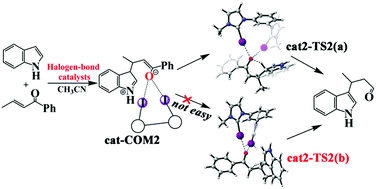
Bidentate halogen bond donor catalysts in organic reactions have attracted great attention in recent years. In this work, the catalytic mechanism of mono/bis(iodoimidazolium) halogen bond donor catalysts in the Michael addition reaction is investigated and bis(iodoimidazolium) halogen bond donor catalysts show a good catalytic performance. Catalyzed by these bidentate catalysts, the studied reaction can occur under mild conditions. The whole catalyzed reaction contains two steps. The formation of a carbon–carbon bond in the first step is a nucleophilic reaction of a C-nucleophile. The following proton transfer process contains two possible reaction pathways. Compared to the direct transfer pathway, the pathway of indirect hydrogen proton transfer has a lower energy barrier, and it is more prone to occur. The halogen bond donor catalysts increase the charge transfer of LP(O) → BD*(C–I), thereby accelerating the charge transfer during the formation of the C–C bond and reducing the reaction energy barrier.


 Please wait while we load your content...
Please wait while we load your content...