Nonadditive ion effects on the coil–globule equilibrium of PNIPAM: a computer simulation study†
Abstract
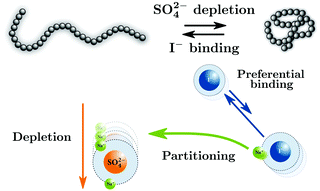
The combined effect of a weakly hydrated and a strongly hydrated anion on the lower critical solution temperature (LCST) of poly(N-isopropylacrylamide)(PNIPAM) is nonadditive (Bruce et al., J. Am. Chem. Soc., 2019, 141, 6609). Herein, we revisit the molecular origin of this effect by performing atomistic molecular dynamics simulations of a 40mer PNIPAM chain in solutions with a fixed concentration of Na2SO4 and an increasing concentration of NaI. Our results show that the nonadditive ion effects on the coil-to-globule transitions of PNIPAM arise due to the interplay between the depletion of the strongly hydrated sulfate ions and the preferential accumulation of the iodide ions on the polymer surface leading to favourable PNIPAM-I− interactions. The depletion of the sulfate ions and the binding of the iodide ions are coupled through the role of the cation leading to a mutual enhancement of both effects. This mutual enhancement effect correlates with the partitioning of the Na+ cations from the counterion cloud of the weakly hydrated iodide ions to the counterion cloud of the strongly hydrated sulfate ions and the corresponding changes in water affinity of the ions.


 Please wait while we load your content...
Please wait while we load your content...