Influence of ionic liquids on the electronic environment of atomically dispersed Ir on (MgO)(100)†
Abstract
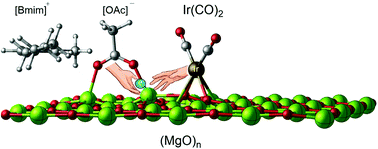
Recently, ionic liquids (ILs) have been used as ligands for single-site Ir(CO)2 complexes bound to metal-oxide supports because of their electron-donor/acceptor capacities. The combined effects of supports and ILs as ligands may pave the way to the tuning of the surrounding electronic properties to increase electron-donor/acceptor efficiency in metal-oxide supported Ir(CO)2 complexes. Herein, we have used Density Functional Theory to model Ir(CO)2 complexes bound to MgO supports with and without the presence of an IL to explain the role of ILs in modifying the electronic structure of the supported complex. Comparison of the ν(CO) band stretching frequencies with experimental results has led to the rationalization of the factors driving the interactions between the IL, the support, and the catalyst as well as the justification of the methodology for further studies.


 Please wait while we load your content...
Please wait while we load your content...