Towards solvent regulated self-activation of N-terminal disulfide bond oxidoreductase-D†
Abstract
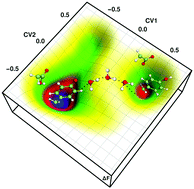
N-terminal disulfide bond oxidoreductase-D (nDsbD), an essential redox enzyme in Gram-negative bacteria, consists of a single disulfide bond (Cys103–Cys109) in its active site. The enzymatic functions are believed to be regulated by an electron transfer mediated redox switching of the disulfide bond, which is vital in controlling bacterial virulence factors. In light of the disulfide bond's inclination towards nucleophilic cleavage, it is also plausible that an internal nucleophile could second the existing electron transfer mechanism in nDsbD. Using QM/MM MD metadynamics simulations, we explore different possibilities of generating an internal nucleophile near the nDsbD active site, which could serve as a fail-over mechanism in cleaving the disulfide bond. The simulations show the formation of the internal nucleophile Tyr42O− (F ≈ 9 kcal mol−1) and its stabilization through the solvent medium. The static gas-phase calculations show that Tyr42O− could be a potential nucleophile for cleaving the S–S bond. Most strikingly, it is also seen that Tyr42O− and Asp68OH communicate with each other through a proton-hole like water wire (F ≈ 12 kcal mol−1), thus modulating the nucleophile formation. Accordingly, we propose the role of a solvent in regulating the internal nucleophilic reactions and the subsequent self-activation of nDsbD. We believe that this could be deterministic while designing enzyme-targeted inhibitor compounds.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...