Quantifiable models for surface protonic conductivity in porous oxides – case of monoclinic ZrO2†
Abstract
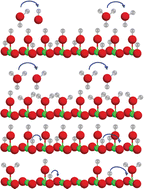
The surface protonic conductivity of porous monoclinic ZrO2 sintered at temperatures in the range 700–1100 °C yielding relative densities of around 60% and grain sizes of approximately 160 nm has been studied using impedance spectroscopy as a function of temperature well below the sintering temperature in wet atmospheres (pH2O = 0.03 bar). The sum of two high-frequency impedance responses is argued to represent surface conductance according to a new model of impedance over curved surfaces. A simple brick layer model is applied to compare the measured macroscopic conductivities with predicted surface conductances. The well-faceted samples sintered at the highest temperatures exhibited activation enthalpies up to 58 kJ mol−1 of surface protonic conduction in wet atmospheres at temperatures above 300 °C. We attribute this to the mobility of dissociated protons over surface oxide ions, and the high preexponential is in good agreement with a model comprising relatively strong dissociative chemisorption. With decreasing sintering temperature, the particles appear more rounded, with less developed facets, and we obtain activation enthalpies of surface protonic conduction in the chemisorbed layer down to around 30 kJ mol−1, with correspondingly smaller preexponentials and an observed 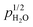 dependency. Supported by the thermogravimetry of adsorption, we attribute this to weaker and more molecular chemisorption on the more randomly terminated less faceted surfaces, providing water layers with fewer dissociated charge carrying protons, but also smaller activation enthalpies of mobility. Below 200 °C, all samples exhibit a strongly inverse temperature dependency characteristic of conduction in the 1st physisorbed layer with increasing coverage. The preexponentials correspond well to the models of physisorption, with dissociation to and proton migration between physisorbed water molecules. The enthalpies fit well to physisorption and with enthalpies of dissociation and proton mobility close to those of liquid water. We have by this introduced models for proton conduction in chemisorbed and physisorbed water on ZrO2, applicable to other oxides as well, and shown that preexponentials are quantitatively assessable in the order-of-magnitude level to discriminate models via a simple brick layer model based topographical analysis of the ceramic microstructure.
dependency. Supported by the thermogravimetry of adsorption, we attribute this to weaker and more molecular chemisorption on the more randomly terminated less faceted surfaces, providing water layers with fewer dissociated charge carrying protons, but also smaller activation enthalpies of mobility. Below 200 °C, all samples exhibit a strongly inverse temperature dependency characteristic of conduction in the 1st physisorbed layer with increasing coverage. The preexponentials correspond well to the models of physisorption, with dissociation to and proton migration between physisorbed water molecules. The enthalpies fit well to physisorption and with enthalpies of dissociation and proton mobility close to those of liquid water. We have by this introduced models for proton conduction in chemisorbed and physisorbed water on ZrO2, applicable to other oxides as well, and shown that preexponentials are quantitatively assessable in the order-of-magnitude level to discriminate models via a simple brick layer model based topographical analysis of the ceramic microstructure.


 Please wait while we load your content...
Please wait while we load your content...