Structural study of 1- and 2-naphthol: new insights into the non-covalent H–H interaction in cis-1-naphthol†
Abstract
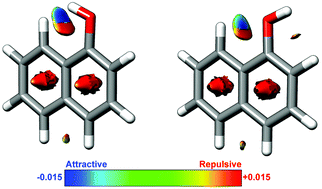
Previous microwave studies of naphthol monomers were supplemented by measuring spectra of all 13C mono-substituted isotopologues of the cis- and trans-conformers of 1-naphthol and 2-naphthol in their natural abundances. The resulting data were utilized to determine substitution structures and so-called semi-experimental effective structures. Results from electronic structure calculations show that the OH group of cis-1-naphthol points ≈6° out of plane, which is consistent with the inertial defect data of cis- and trans-1-naphthol. The non-planarity of cis-1-naphthol is a result of a close-contact H-atom–H-atom interaction. This type of H–H interaction has been the subject of much controversy in the past and we provide here an in-depth theoretical analysis of it. The naphthol system is particularly well-suited for such analysis as it provides internal standards with its four different isomers. The methods used include quantum theory of atoms in molecules, non-covalent interactions, independent gradient model, local vibrational mode, charge model 5, and natural bond orbital analyses. We demonstrate that the close-contact H–H interaction is neither a purely attractive nor repulsive interaction, but rather a mixture of the two.


 Please wait while we load your content...
Please wait while we load your content...