Reversible photo control of proton chemistry
Abstract
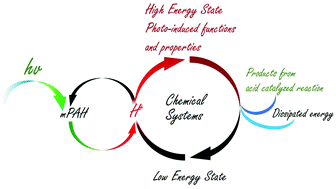
Spatial, temporal, and remote control of proton chemistry can be achieved by using photoacids, which are molecules that transform from weak to strong acids under light. Most of proton chemistry is driven by a high concentration of protons ([H+]), which is difficult to obtain using excited-state photoacids. Metastable-stable state photoacids (mPAHs) can reversibly generate a high [H+] under visible light with a moderate intensity. It has been widely applied in different fields, e.g. fueling dissipative assemblies, driving molecular machines, controlling organic reactions, powering nanoreactors, curing diseases, manipulating DNA and proteins, developing smart materials, capturing carbon dioxide in air etc. This article compares mPAH with excited-state photoacid as well as common acids e.g. HCl to explain its advantages. Recent studies on the thermal dynamics, kinetics, and photoreaction of mPAHs are reported. The advantages and disadvantages of the three types of mPAHs, i.e. merocyanine, indazole, and TCF mPAHs, are compared with regard to photo-induced [H+], switching rate, and other properties. The mechanisms of controlling or driving functional systems, which involve acid–base reactions, acid catalyzed reactions, ionic bonding, coordination bonding, hydrogen bonding, ion exchange, cation–π interaction, solubility, swellability, permeability, and pH change in biosystems, are described. Applications of mPAHs in the chemical, material, energy, biotechnology and biomedical fields published in the past 5 years are reviewed. Prospects in the development and application of mPAHs are discussed.
- This article is part of the themed collection: PCCP Reviews


 Please wait while we load your content...
Please wait while we load your content...