Aromaticity-promoted CS2 activation by heterocycle-bridged P/N-FLPs: a comparative DFT study with CO2 capture†
Abstract
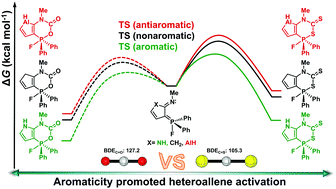
Carbon dioxide (CO2) capture has attracted considerable attention from both experimental and theoretical chemists. In comparison, carbon disulfide (CS2) activation is less developed. Here, we carry out a thorough comparative density functional theory study to examine the reaction mechanisms of CS2 activation by five-membered heterocycle-bridged P/N frustrated Lewis pairs (FLPs). Calculations suggest that despite a weaker carbon–sulfur bond, all the CS2 activations have higher reaction barriers than the CO2 capture, which could be attributed to electrostatic repulsion between FLPs and CS2 caused by the reversed polarity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S in CS2 rather than the electrostatic attraction in CO2 capture. In addition, aromaticity is found to play an important role in CS2 capture as it stabilizes both the transition states and products in heterocycle-bridged FLPs. All these findings could be useful for experimentalists to realize small molecule activations by FLPs.
S in CS2 rather than the electrostatic attraction in CO2 capture. In addition, aromaticity is found to play an important role in CS2 capture as it stabilizes both the transition states and products in heterocycle-bridged FLPs. All these findings could be useful for experimentalists to realize small molecule activations by FLPs.


 Please wait while we load your content...
Please wait while we load your content...