Modulating the spectroscopy and dynamics of a proton-transfer dye by functionalizing with phenyl groups†
Abstract
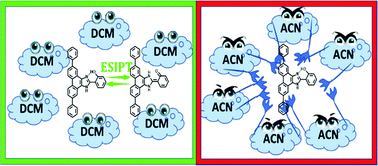
Molecules undergoing excited-state proton transfer (ESPT) reactions are among the most interesting systems from spectroscopic and photophysical viewpoints. These molecules can be further functionalized with electron donating or accepting groups, inducing intramolecular charge transfer (ICT) events, which might be coupled to the ESIPT ones, conferring them with different spectroscopic and photophysical properties, which can be essential to implement the related materials in many key scientific and technological fields. Here, we report new benzimidazole derivatives that are functionalized with a phenyl group, 2-(5,10-diphenyl-1H-phenanthro[9,10-d]imidazol-2-yl)phenol (DP-HPPI), and its methylated equivalent, 2-(2-methoxyphenyl)-5,10-diphenyl-1H-phenanthro[9,10-d]imidazole (DP-MPPI). The results prove that these molecules in solutions undergo an ultrafast ICT (400–700 fs) reaction. Additionally, DP-HPPI also undergoes a reversible ESIPT process in dichloromethane. However, this is precluded in acetonitrile due to the involvement of intermolecular H-bonds in this solvent. These results provide key insights into the development of proton-transfer materials with bespoke spectral and photodynamical properties.
- This article is part of the themed collection: Developments in Ultrafast Spectroscopy


 Please wait while we load your content...
Please wait while we load your content...