Interconversion between Lewis and Brønsted–Lowry acid sites on vanadia-based catalysts†
Abstract
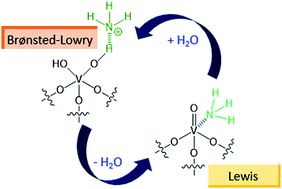
Lewis acid sites (LAS) and Brønsted–Lowry acid sites (BAS) play key roles in many catalytic processes, particularly in the selective catalytic reduction (SCR) of nitrogen oxides with ammonia. Here we show that temperature, gas feed, and catalyst composition affect the interplay between LAS and BAS on vanadia-based materials under SCR-relevant conditions. While different LAS typically manifest as a single collective peak in the steady-state spectra, their individual signals could be isolated through the increased sensitivity of transient experimentation. Furthermore, water could increase BAS not just by converting pre-existing LAS, but also by generating spontaneously new acid sites. These results pave the way for understanding the relationship between LAS and BAS, and how their ratio determines the reactivity of vanadia-based catalysts not just in SCR but in other chemical transformations as well.


 Please wait while we load your content...
Please wait while we load your content...