Low-temperature heat capacity and pseudorotation in 2-methyltetrahydrofuran†
Abstract
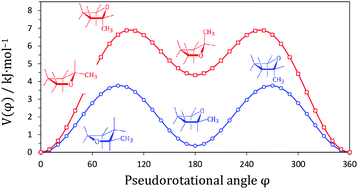
The heat capacity and phase transitions of 2-methyltetrahydrofuran in the temperature range from 7 to 350 K were measured using adiabatic calorimetry. The smoothed molar thermodynamic functions in the condensed state were determined on the basis of these measurements. The thermodynamic functions of the formation were also calculated. The gas-phase entropy at 298.15 K was obtained using the entropy of crystal 2-methyltetrahydrofuran, entropy of fusion and entropy of vaporization. This entropy value was used to clarify some aspects of the discrepancies between the interpretations of pseudorotation in 2-methyltetrahydrofuran. An extended quantum chemical study of pseudorotation in 2-methyltetrahydrofuran was undertaken to provide additional insight into the conformational features of a molecule. The contribution of pseudorotation to the entropy was calculated for different pseudorotational potentials constructed using various theoretical models. The experimental entropy value is in best agreement with intramolecular conversion between two low energy conformers connected by the transition state with an energy of about 4 kJ mol−1. The standard thermodynamic properties of 2-methyltetrahydrofuran in the gaseous state (T = 100 K to 1500 K) were calculated using experimental and theoretical molecular parameters.


 Please wait while we load your content...
Please wait while we load your content...