O-Acetylated sugars in the gas phase: stability, migration, positional isomers and conformation†
Abstract
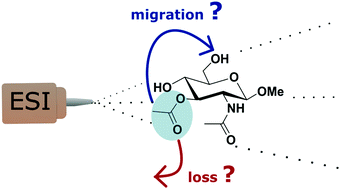
O-Acetylations are functional modifications which can be found on different hydroxyl groups of glycans and which contribute to the fine tuning of their biological activity. Localizing the acetyl modifications is notoriously challenging in glycoanalysis, in particular because of their mobility: loss or migration of the acetyl group may occur through the analytical workflow. Whereas migration conditions in the condensed phase have been rationalized, little is known about the suitability of Mass Spectrometry to retain and resolve the structure of O-acetylated glycan isomers. Here we used the resolving power of infrared ion spectroscopy in combination with ab initio calculations to assess the structure of O-acetylated monosaccharide ions in the gaseous environment of a mass analyzer. N-Acetyl glucosamines were synthetized with an O-acetyl group in positions 3 or 6, respectively. The protonated ions produced by electrospray ionization were observed by mass spectrometry and their vibrational fingerprints were recorded in the 3 μm range by IRMPD spectroscopy (InfraRed Multiple Photon Dissociation). Experimentally, the isomers show distinctive IR fingerprints. Additionally, ab initio calculations confirm the position of the O-acetylation and resolve their gas phase conformation. These findings demonstrate that the position of O-acetyl groups is retained through the transfer from solution to the gas phase, and can be identified by IRMPD spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...