Reaction mechanism of an intracluster SN2 reaction induced by electron capture†
Abstract
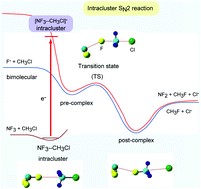
Bimolecular nucleophilic substitution (SN2) reactions have been widely investigated from both experimental and theoretical points of view because they represent one of the simplest organic reactions. Most studies on SN2 reactions have been focused on bimolecular collision. In contrast, information on intracluster SN2 reactions is limited. In this study, an intracluster SN2 reaction of NF3–CH3Cl triggered by electron attachment was investigated using a direct ab initio molecular dynamics (AIMD) method. In the structure of NF3–CH3Cl, the N–F bond in NF3 is oriented collinearly toward the carbon atom of CH3Cl. After electron capture by NF3–CH3Cl, the F− ion that is generated from the (NF3)− moiety collides with the carbon atom of CH3Cl. The intracluster SN2 reaction occurs as follows: (NF3–CH3Cl)− (electron capture state) → NF2–(F−)–CH3Cl (pre-reaction complex) → transition state (TS) → NF2–CH3F–Cl− (post-reaction complex) → NF2 + CH3F + Cl− (product state). The reaction energy is efficiently transferred to the translational mode of Cl−, and the Cl− ion with a high translational energy is then removed from the system. This energy is significantly larger than that of Cl− formed in the bimolecular SN2 reaction (F− + CH3Cl). The reaction mechanism is discussed based on the theoretical results.


 Please wait while we load your content...
Please wait while we load your content...