The radicals of quercetin-derived antioxidants in Triton X-100 micelles†
Abstract
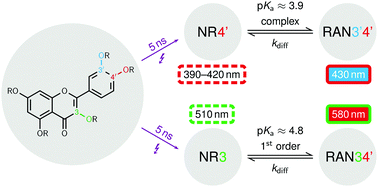
We have employed photoionization with a pulsed laser (5 ns, 355 nm) as a direct access to the radicals of quercetin, five of its monoethers and three of its diethers in nonionic micelles. On a submicrosecond timescale, the first detectable intermediates are neutral radicals NRx, which can then be deprotonated to give radical anions RANxy, where x and y denote the phenoxyl positions bearing spin and/or charge. Alkylation at oxygen x blocks the formation of NRx and RANxy but barely changes the spectra of all other structurally possible radical isomers. Through systematic comparison, this allowed unambiguous radical identification and spectral assignment by experiment in all cases: NR3 is preferred over NR4′, all other NRx are negligible; NR3 and NR4′ are deprotonated at oxygens 4′ and 3′, respectively, unless barred by substitution, or at oxygen 7. As a caveat, B3LYP calculations on the radicals with a 6-311++g(2d,2p) basis set and a PCM solvation model gave only partially correct energy orderings and spectra, the former most likely due to an inability fully to describe the intramolecular hydrogen bonds and the latter possibly due to spin contamination. The favored deprotonation of NR3 is associated with a typical pKa of 4.8 and first-order kinetics, that of NR4′ with a pKa of 3.9 and complex kinetics, suggesting NR3′ as a fleeting intermediate. Both reverse reactions are diffusion controlled.


 Please wait while we load your content...
Please wait while we load your content...