CO2-Activation by size-selected tantalum cluster cations (Ta1–16+): thermalization governing reaction selectivity†
Abstract
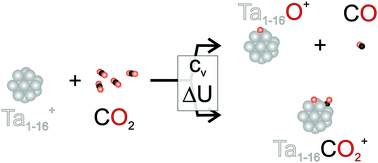
The reactions of tantalum cluster cations of different sizes toward carbon dioxide are studied in an ion trap under multi-collisional conditions. For all sizes studied, consecutive reactions with several CO2 molecules are observed. This reveals two different pathways, namely oxide formation and the pickup of an entire molecule. Supported by calculations of the thermochemistry of TanO+ formation upon reaction with CO2, changes in the branching ratios at a particular cluster size are related to heat effects due to the vibrational heat capacity of the clusters and the exothermicity of the reaction.


 Please wait while we load your content...
Please wait while we load your content...