Cavity-based free energy analysis of osmolyte effects on protein denaturation†
Abstract
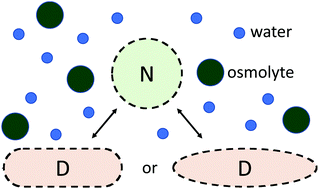
Experimental measurements of the thermal effects of the same osmolytes on two different globular proteins, C-reactive protein (CRP) and tumor necrosis factor alpha (TNFα), have shown that quantifying the change in the denaturing temperature leads to some results that are unique to each protein. In order to find osmolyte-dependent parameters that can be applied more consistently from protein to protein, this work considers, instead, the overall free energy change associated with that denaturation using coarse-grained models. This is enabled by using theoretical fluid equations that take into account the exclusion of water and osmolyte from the volume occupied by the protein in both its native and denatured forms. Assuming ideal geometric models of the two protein states whose sizes are based on the protein's surface area in each form, and taking into account the density of the aqueous osmolyte solution, the free energy change due to the change in geometry can be calculated. The overall change in free energy of the system is found from that quantity and other protein- and osmolyte-specific parameters, which are determined using the experimental concentration and temperature results. We find that these fitted parameters accurately reproduce experimental results and also show consistent patterns from protein to protein. We also consider two different model geometries of the denatured protein and find little impact on the use of one or the other. Defining the effects of the osmolyte in terms of free energy also allows for prediction of overall phase change behavior, including cold denaturation.


 Please wait while we load your content...
Please wait while we load your content...