Pressure-stabilized hexafluorides of first-row transition metals†
Abstract
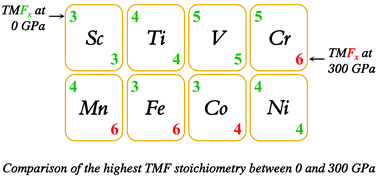
Fluorine chemistry was demonstrated to show the importance of stretching the limits of chemical synthesis, oxidation state, and chemical bonding at ambient conditions. Thus far, the highest fluorine stoichiometry of a neutral first-row transition-metal fluoride is five, in VF5 and CrF5. Pressure can stabilize new stoichiometric compounds that are inaccessible at ambient conditions. Here, we attempted to delineate the fluorination limits of first-row transition metals at a high pressure through first-principles swarm-intelligence structure searching simulations. Besides reproducing the known compounds, our extensive search has resulted in a plethora of unreported compounds: CrF6, MnF6, FeF4, FeF5, FeF6, and CoF4, indicating that the application of pressure achieves not only the fluorination limit (e.g., hexafluoride) but also the long-sought bulky tetrafluorides. Our current results provide a significant step forward towards a comprehensive understanding of the fluorination limit of first-row transition metals.


 Please wait while we load your content...
Please wait while we load your content...