Bis-isonicotinoyl linkers containing polyaromatic scaffolds: synthesis, structure and spectroscopic properties†
Abstract
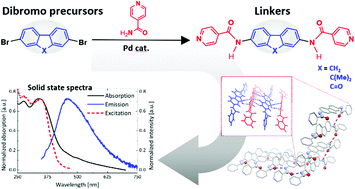
In this study, a new series of extended linkers containing different polyaromatic chromophores (biphenyl, naphthalene, anthracene, fluorene, 9,9-dimethylfluorene and fluorenone) functionalized with isonicotinoyl moieties have been synthesized by Pd-catalyzed cross-coupling reactions involving isonicotinamide and the appropriate aromatic dibromide. The optimized protocol led to the isolation of the target molecules in good yield and with high purity. These were characterized by 1H NMR, FTIR, MS, and elemental analysis and their solid state structures were solved by single-crystal X-ray diffraction analysis. Electronic absorption and emission spectra were collected both in solution (DMF) and in the solid state. TDDFT calculations were carried out to investigate the effect of the isonicotinoyl moieties on the spectral features of the central chromophores. Although in solution only the linker containing a fluorenone scaffold shows a weak fluorescence, all the isolated linkers turned out to be fluorescent in the solid state, thus paving the way for their use for the fabrication of fluorescent MOFs.


 Please wait while we load your content...
Please wait while we load your content...