An evaluation of the capacitive behavior of supercapacitors as a function of the radius of cations using simulations with a constant potential method†
Abstract
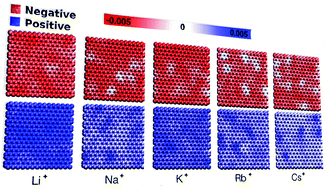
We report on the atomistic molecular dynamics, applying the constant potential method to determine the structural and electrostatic interactions at the electrode–electrolyte interface of electrochemical supercapacitors as a function of the cation radius (Cs+, Rb+, K+, Na+, Li+). We find that the electrical double layer is susceptible to the size, hydration layer volume, and cations’ mobility and analyzed them. Besides, the transient potential shows an increase in magnitude and length as a function of the monocation size, i.e., Cs+ > Rb+ > K+ > Na+ > Li+. On the other hand, the charge distribution along the electrode surface is less uniform for large monocations. Nonetheless, the difference is not observed as a function of the radius of the cation for the integral capacitance. Our results are comparable to studies that employed the fixed charge method for treating such systems.


 Please wait while we load your content...
Please wait while we load your content...