The kinetics of the reactions of Br atoms with the xylenes: an experimental and theoretical study†
Abstract
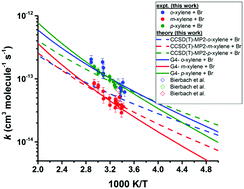
This work reports the temperature dependence of the rate coefficients for the reactions of atomic bromine with the xylenes that are determined experimentally and theoretically. The experiments were carried out in a Pyrex chamber equipped with fluorescent lamps to measure the rate coefficients at temperatures from 295 K to 346 K. Experiments were made at several concentrations of oxygen to assess its potential kinetic role under atmospheric conditions and to validate comparison of our rate coefficients with those obtained by others using air as the diluent. Br2 was used to generate Br atoms photolytically. The relative rate method was used to obtain the rate coefficients for the reactions of Br atoms with the xylenes. The reactions of Br with both toluene and diethyl ether (DEE) were used as reference reactions where the loss of the organic reactants was measured by gas chromatography. The rate coefficient for the reaction of Br with diethyl ether was also measured in the same way over the same temperature range with toluene as the reference reactant. The rate coefficients were independent of the concentration of O2. The experimentally determined temperature dependence of the rate coefficients of these reactions can be given in the units cm3 molecule−1 s−1 by: o-xylene + Br, log10(k) = (−10.03 ± 0.35) − (921 ± 110)/T; m-xylene + Br, log10(k) = (−10.78 ± 0.09) − (787 ± 92/T); p-xylene + Br, log10(k) = (−9.98 ± 0.39) − (956 ± 121)/T; diethyl ether + Br, log10(k) = (−7.69 ± 0.55) − (1700 ± 180)/T). This leads to the following rate coefficients, in the units of cm3 molecule−1 s−1, based on our experimental measurements: o-xylene + Br, k(298 K) = 7.53 × 10−14; m-xylene + Br, k(298 K) = 3.77 × 10−14; p-xylene + Br, k(298 K) = 6.43 × 10−14; diethyl ether + Br, k(298 K) = 4.02 × 10−14. Various ab initio methods including G3, G4, CCSD(T)/cc-pV(D,T)Z//MP2/aug-cc-pVDZ and CCSD(T)/cc-pV(D,T)Z//B3LYP/cc-pVTZ levels of theory were employed to gain detailed information about the kinetics as well as the thermochemical quantities. Among the ab initio methods, the G4 method performed remarkably well in describing the kinetics and thermochemistry of the xylenes + Br reaction system. Our theoretical calculations revealed that the reaction of Br atoms with the xylenes proceeds via a complex forming mechanism in an overall endothermic reaction. The rate determining step is the intramolecular rearrangement of the pre-reactive complex leading to the post-reactive complex. After lowering the relative energy of the corresponding transition state by less than 1.5 kJ mol−1 for this step in the reaction of each of the xylenes with Br, the calculated rate coefficients are in very good agreement with the experimental data.


 Please wait while we load your content...
Please wait while we load your content...