A computational study of the HO2 + SO3 → HOSO2 + 3O2 reaction catalyzed by a water monomer, a water dimer and small clusters of sulfuric acid: kinetics and atmospheric implications†
Abstract
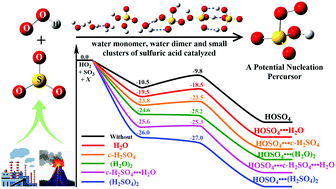
Herein, the reaction mechanisms and kinetics for the HO2 + SO3 → HOSO2 + 3O2 reaction catalyzed by a water monomer, a water dimer and small clusters of sulfuric acid have been studied theoretically by quantum chemical methods and the Master Equation/Rice–Ramsperger–Kassel–Marcus (ME/RRKM) rate calculations. The calculated results show that when H2O is introduced into the HO2 + SO3 reaction, it not only enhances the stability of the reactant complexes by 9.0 kcal mol−1 but also reduces the energy of the transition state by 8.7 kcal mol−1. As compared with H2O, catalysts (H2O)2, H2SO4, H2SO4⋯H2O and (H2SO4)2 are more effective energetically, which not only results from a higher binding energy of 21.3–26.0 kcal mol−1 for the reactant complexes but also from a reduction of the energy of the transition states by 8.6–17.2 kcal mol−1. Effective rate constant calculations show that, as compared with H2O, catalysts (H2O)2, H2SO4, H2SO4⋯H2O and (H2SO4)2 can never become more efficient catalysts within the altitude range of 0–15 km due to their relatively lower concentrations. Besides, at 0 km altitude, the enhancement factor 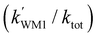 for the H2O and (k′WD1/ktot) (H2O)2-assisted HO2 + SO3 reaction within the temperature range of 280–320 K was respectively calculated to be 0.31%–0.34% and 0.16%–0.27%, while the corresponding enhancement factor of H2O and (H2O)2 at higher altitudes of 5–15 km was respectively found only 0.002%–0.12% and 0.00001%–0.022%, indicating that the contributions of H2O and (H2O)2 are not apparent in the gas-phase reaction of HO2 with SO3 especially at higher altitude. Overall, the present work will give a new insight into how a water monomer, a water dimer and small clusters of sulfuric acid catalyze the HO2 + SO3 → HOSO2 + 3O2 reaction.
for the H2O and (k′WD1/ktot) (H2O)2-assisted HO2 + SO3 reaction within the temperature range of 280–320 K was respectively calculated to be 0.31%–0.34% and 0.16%–0.27%, while the corresponding enhancement factor of H2O and (H2O)2 at higher altitudes of 5–15 km was respectively found only 0.002%–0.12% and 0.00001%–0.022%, indicating that the contributions of H2O and (H2O)2 are not apparent in the gas-phase reaction of HO2 with SO3 especially at higher altitude. Overall, the present work will give a new insight into how a water monomer, a water dimer and small clusters of sulfuric acid catalyze the HO2 + SO3 → HOSO2 + 3O2 reaction.


 Please wait while we load your content...
Please wait while we load your content...