Aerobic photolysis of methylcobalamin: unraveling the photoreaction mechanism†
Abstract
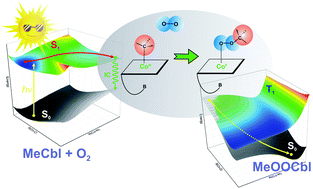
The photo-reactivity of cobalamins (Cbls) is influenced by the nature of axial ligands and the cofactor's environment. While the biologically active forms of Cbls with alkyl axial ligands, such as methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl), are considered to be photolytically active, in contrast, the non-alkyl Cbls are photostable. In addition to these, the photolytic properties of Cbls can also be modulated in the presence of molecular oxygen, i.e., under aerobic conditions. Herein, the photoreaction of the MeCbl in the presence of oxygen has been explored using density functional theory (DFT) and time-dependent DFT (TD-DFT). The first stage of the aerobic photoreaction is the activation of the Co–C bond and the formation of the ligand field (LF) electronic state through the displacement of axial bonds. Once the photoreaction reaches the LF excited state, three processes can occur: namely the formation of OO–CH3 through the reaction of CH3 with molecular oxygen, de-activation of the {Im⋯[CoII(corrin)]⋯CH3}+ sub-system from the LF electronic state by changing the electronic configuration from (dyz)1(dz2)2 to (dyz)2(dz2)1 and the formation of the deactivation complex (DC) complex via the recombination of OO–CH3 species with the de-excited [CoII(corrin)] system. In the proposed mechanism, the deactivation of the [CoII(corrin)] subsystem may coexist with the formation of OO–CH3, followed by immediate relaxation of the subsystems in the ground state. Moreover, the formation of the OO–CH3 species followed by the formation of the {[CoIII(corrin)]–OO–CH3}+ complex stabilizes the system compared to the reactant complex.


 Please wait while we load your content...
Please wait while we load your content...