Varied role of organic carboxylate dizwitterions and anionic donors in mixed-ligand uranyl ion coordination polymers†
Abstract
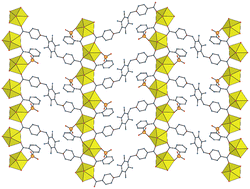
The dizwitterionic dicarboxylate ligands 1,1′-[(2,3,5,6-tetramethylbenzene-1,4-diyl)bis(methylene)]bis(pyridin-1-ium-4-carboxylate) (pL) and 1,1′-[(2,3,5,6-tetramethylbenzene-1,4-diyl)bis(methylene)]bis(pyridin-1-ium-3-carboxylate) (mL) have been reacted with uranyl cations under solvo-hydrothermal conditions to generate a series of five complexes containing also additional anionic donors. [UO2(pL)(H2PM)(H2O)2]·DMA·H2O (1), where H4PM is pyromellitic acid and DMA is dimethylacetamide, and [(UO2)2(pL)3(3-SB)2]·8H2O (2), where 3-SB2− is 3-sulfobenzoate, crystallize as monoperiodic coordination polymers, linear or including dinuclear rings, respectively, in which the pL ligands are bridging and the anionic species are merely decorating and involved in hydrogen bonding. pL connects four metal cations in [(UO2)2(pL)(2-SB)2]·1.5H2O (3) and, associated with the chelating and bridging 2-sulfobenzoate (2-SB2−) ligand, it gives a diperiodic network with the kgm topology. [(UO2)3(mL)(O)2(OH)(H2O)](NO3)0.8Cl0.2·3H2O (4) contains oxo- and hydroxo-bridged ribbon-like chains connected by mL linkers to form a cationic diperiodic network. In contrast, two independent, polyanionic and polycationic networks in a 2 : 1 ratio are formed in [(UO2)2(mL)3(H2O)2][(UO2)2(TDC)3]2·10H2O (5), involving either mL or TDC2− (2,5-thiophenedicarboxylate) ligands; both networks have the hcb topological type, albeit with very different cell shapes.


 Please wait while we load your content...
Please wait while we load your content...