A new {Cu3–Gd2} cluster as a two-in-one functional material with unique topology acting as a refrigerant and adsorbent for cationic dye†
Abstract
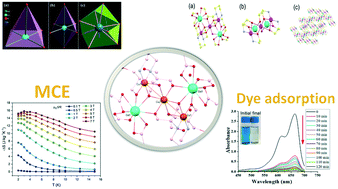
A new heterometallic cluster system, [Cu3Gd2(H3btp)2(OAc)6]·5H2O {Cu3–Gd2}, is designed by employing 1,3-bis(tris(hydroxymethyl)methylamino)propane (H6btp) as a ligand. The cluster is characterized by FTIR, TGA, PXRD, SCXRD and topological analyses. The crystallography ascertains the presence of two Cu2+ in square pyramidal and one Cu2+ in octahedral environments while the two Gd3+ ions are in a distorted capped square antiprismatic geometry. The ligand is coordinated in an unusual tri-deprotonated form (H3btp3−). The overall cluster represents a rare example of unique topology [1,2 M3-1]. The M(H) plot recorded at 2 K shows the presence of antiferromagnetic interactions. The cluster exhibits a maximum change in entropy of 15.84 J kg−1 K−1 at 3 K for H = 7 T thus acting as a new class of magnetic molecular cooler. Furthermore, free –OH groups in the architecture of the cluster facilitate the adsorption of aromatic cationic dye, methylene blue (MB), selectively owing to strong O–H⋯π interactions with good recyclability at ambient pH and temperature. This cluster is a rare example among a few reported discrete units showing structure–property relation for adsorption of MB. The present work opens new doors for designing exotic materials having a tunable magnetocaloric effect (MCE) as well as cationic dye adsorption with notable efficacy and presents the first example of a dual functional material as a coolant and an adsorbent.


 Please wait while we load your content...
Please wait while we load your content...