Sustainable solid form screening: mechanochemical control over nucleobase hydrogen-bonded organic framework polymorphism†
Abstract
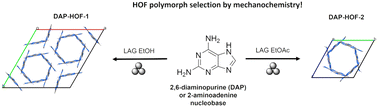
With an increasing need to make preparative chemistry more sustainable, mechanochemistry receives much attention. It is attractive for synthesizing open coordination or covalent frameworks, offering an unprecedented level of synthetic control while reducing solvent usage and frequently accessing products not available by conventional synthetic methods. Here, we present a sustainable solid form screening of 2,6-diaminopurine (DAP) nucleobase, an important hydrogen-bonded framework building block. DAP is well known for treating genetic diseases and occurring as a nucleobase in viral DNA, yet its solid-state chemistry is unknown. With a total solvent consumption of 0.6 mL, we discovered five new DAP solid forms by mechanochemical and thermal routines, including a polymorphic pair of DAP hydrogen-bonded organic frameworks (HOFs). Two DAP-HOF polymorphs formed selectively by choosing the liquid additive in liquid-assisted grinding (LAG). Monitoring by in situ Raman spectroscopy revealed different formation profiles for each DAP-HOF polymorph, and nitrogen adsorption isotherms confirmed their permanent porosities. The density functional theory (DFT) analysis established the relative thermodynamic stability of crystallographically characterized DAP solid forms. Our results demonstrate the ability of mechanochemistry to control HOF polymorphism, which might be critical for their properties and subsequent applications. In a broader perspective, this work opens the door for sustainable studies on nucleobase-inspired HOF materials.
- This article is part of the themed collection: #RSCPoster Conference


 Please wait while we load your content...
Please wait while we load your content...