Solvent-induced phase transformations of ZIF-L to ZIF-8 and their derivatives' gas-sensing properties†
Abstract
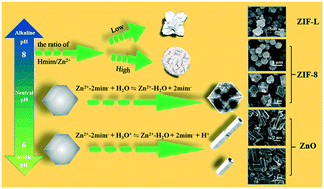
A detailed study of topological phase transition is essential for guiding the controlled synthesis of materials. Herein, a series of zeolitic-imidazolate frameworks (ZIFs) and their derived metal oxides with tailored morphologies and adjustable phases from ZIF-L to ZIF-8 to ZnO were reported. More significantly, the phase transformation and morphology control of the product could be achieved by simply changing the molar/volume ratio of the reagents without adding any surfactant, and the growth mechanism was systemically investigated. Benefiting from their unique structural advantage, when fabricating gas sensors, all of the products and their derivatives exhibited excellent gas-sensing selectivity and rapid response/recovery times toward acetone vapor. In addition, the gas-sensing mechanism and the causes for the gas-sensing performance discrepancy in different sensors were also studied. The results showed that the oxygen vacancy (OV) content, structures, and morphologies of the product were closely related to their comparable sensitivities. The phase transition and morphology evolution of ZIFs and ZnO in this study might bring a deep understanding of the metal–organic framework crystallization process, which is expected to help the development of more precise control strategies for MOF-based materials with customized functional properties.
- This article is part of the themed collection: Editor’s Collection: Engineering Zeolitic Imidazolate Framework-8-based materials


 Please wait while we load your content...
Please wait while we load your content...