Polymorphism and photoluminescence seen in (2-amino-5-chloropyridine)·(9-anthracenecarboxylic acid)·(trinitrobenzene): a further example of the salt-cocrystal continuum observed by virtue of isolating multiple crystal forms†
Abstract
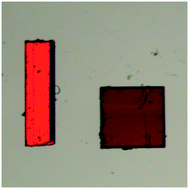
Two polymorphic forms of the ternary adduct (2-amino-5-chloropyridine)·(9-anthracenecarboxylic acid)·(trinitrobenzene) were isolated. In both forms 9-anthracenecarboxylic acid displays a charge-transfer interaction with trinitrobenzene. Form I exhibits proton transfer from the carboxylic acid to the pyridine and forms a ternary molecular salt, while form II forms a ternary co-crystal. Form I crystallises out in acetonitrile, whilst form II crystalizes out in methanol. The two forms occur as concomitant pairs when crystallised from a variety of solvents. Form I crystallises as orange needles and form II crystallises as red blocks. Differential scanning calorimetry indicates that form II is the thermodynamically most stable form, and form I is the kinetically favoured form. Form I, being a molecular salt, luminesces whilst form II, being a cocrystal, does not luminesce. The two polymorphs are a further illustration of the salt/cocrystal continuum.


 Please wait while we load your content...
Please wait while we load your content...