Solid-state fluorescence of a quasi-isostructural polymorphic biphenyl based Michael addition product†
Abstract
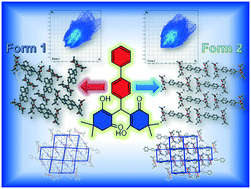
Polymorphic materials have gained significant attention owing to their fascinating physicochemical properties. Herein, a biphenyl based Michael addition product (compound A) with an active methylene group (dimedone) was synthesized. Compound A displayed aggregation-induced emission in an ethanol–water system and in the solid state owing to its highly twisted conformation due to two bulky dimedone groups connected to a sp3 hybridized C atom. It is dimorphic in nature (forms 1 and 2) with the two forms having identical crystal packing densities (the calculated density is 1.201 g cm−3). Form 1 was solved in the P21/c monoclinic space group, whereas form 2 was solved in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) triclinic space group. The quasi-isostructural nature of the two polymorphic systems of the synthesized compound resulted in identical photo-physical behaviours.
triclinic space group. The quasi-isostructural nature of the two polymorphic systems of the synthesized compound resulted in identical photo-physical behaviours.
- This article is part of the themed collection: Editor’s Collection: The application of quantum crystallography to solid-state pharmaceuticals


 Please wait while we load your content...
Please wait while we load your content...