Synergetic effect of photocatalysis and peroxymonosulfate activation by MIL-53Fe@TiO2 on efficient degradation of tetracycline hydrochloride under visible light irradiation†
Abstract
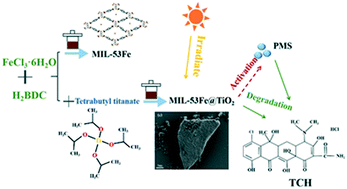
MIL-53Fe was prepared by a simple solvothermal method, and the MIL-53Fe photocatalysts showed low photocatalytic activity for the degradation of tetracycline hydrochloride under visible light irradiation. After combination with TiO2, the photocatalytic activity of MIL-53Fe was enhanced obviously. The enhanced photocatalytic activity of the MIL-53Fe@TiO2 photocatalysts could be attributed to the effective separation of the photogenerated e−/h+ pairs. In order to further enhance the degradation efficiency of MIL-53Fe@TiO2, it was used to activate peroxymonosulfate to degrade tetracycline hydrochloride. The influencing factors on the activation of peroxymonosulfate to degrade tetracycline hydrochloride were investigated in detail. The mechanism of MIL-53Fe@TiO2 activation of peroxymonosulfate was investigated by radical quenching experiments, which proved that holes and sulfate radicals controlled the degradation of tetracycline hydrochloride. Cycle experiments showed that the photodegradation efficiency did not change obviously after the catalyst was recycled three times. This study provided a new way that the synergetic effect of photocatalysis and peroxymonosulfate activation by transition metal was used for efficient photocatalytic degradation in the field of antibiotic removal, hoping to provide new ideas for the development of metal–organic frameworks.


 Please wait while we load your content...
Please wait while we load your content...