Stabilizing nonnative polymorphs at the nanoscale as surface energy is inversely correlated to bulk energies
Abstract
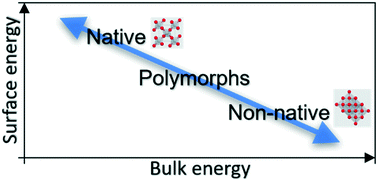
We demonstrate a correlation of utility in stabilizing different material polymorphs. To circumvent the challenges of ranking materials in terms of metastability and/or kinetic stability, we utilize a structural nomenclature of “native” and “nonnative” polymorphs. The native polymorph is the thermodynamically most stable bulk crystal polymorph—the one with the lowest/most negative Gibbs free energy of formation. All nano or bulk crystals with a discrete translational symmetry different from the native phase are termed nonnative phases. From the analysis of relevant experiments and computations, we demonstrate the correlation that a nonnative phase has higher surface stability than the corresponding native phase. We then evaluate the trend in surface energy per bond for periodic table elements and find that nonnative polymorphic surfaces are consistently more stable than native structures, suggesting a preferential stabilization of the nonnative phases on the nanoscale. These heuristics for phase selection facilitate the engineering of properties of many material-centric devices.


 Please wait while we load your content...
Please wait while we load your content...