Design and synthesis of coordination polymers with Cu(ii) and heterocyclic N-oxides†
Abstract
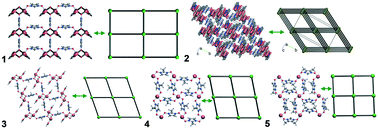
The relations of coordination network connectivity with coordination properties of heterocyclic N-oxides, Cu(I,II), and co-ligands were discussed based on the comparative analysis of 623 structures extracted from the Cambridge Structural Database. The structures were sorted into three main groups of combinations of the topological descriptors of the heterocyclic N-oxide ligand leading to the formation of molecular complexes, polymeric structures, and structures with clusters {CuxOy}. Only two, κ1 and κ2, coordination types were found for the N-oxide group. The κ2 type contributes to the formation of dimeric, trimeric, and rod secondary building units, attractive for the design of more stable 2D and 3D coordination polymers. Other functional groups of the heterocyclic N-oxides and co-ligands mainly result in increasing the connectivity of the complex, while terminal ligands restrict the connectivity. Based on the topological relations, five new coordination compounds of Cu(II) with 1-hydroxoimidazole-3-oxide (HL1), 2-methyl-1-hydroxoimidazole-3-oxide (HL2), and 4,5-dimethyl-1-hydroxoimidazole-3-oxide (HL3) were designed and synthesized: [Cu(H2O)(HCO2)(L1)]·0.5H2O (1), [Cu3(L1)4(CH3CO2)2] (2), [Cu(H2O)(HCO2)(L2)]·H2O (3), [Cu(L2)2] (4), and [Cu(L3)2] (5). The new compounds were studied by X-ray structural analysis, powder X-ray diffraction, and Fourier transform infrared spectroscopy. The N-oxide groups of the ligands are deprotonated in all the compounds and coordinated with 1 or 2 metal atoms, while the 1-hydroxoimidazole-3-oxides are coordinated in μ (1, 4, and 5), μ3 (2 and 3), and μ4 (1) bridging modes. Two (1 and 3) and one (2) compounds contain, respectively, di- and trinuclear secondary building units. Four (1, 3, 4, and 5) and one (2) compounds exhibit, respectively, 2D and 3D architectures of the topological types sql and pcu. It was found that the underlying topology pcu has not been observed before for such compounds, and the content of the ligand in the complex can be tuned by the choice of a co-ligand. The exfoliation of three new 2D coordination polymers was shown by liquid phase sonication and the Tyndal effect.


 Please wait while we load your content...
Please wait while we load your content...