Conformational flexibility and substitution pattern lead to polymorphism of 3-methyl-2-(phenylamino)benzoic acid†
Abstract
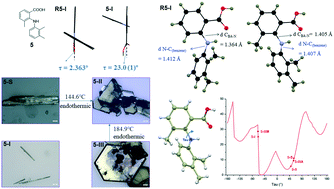
To probe the effect of substitution on the benzoic acid ring on the polymorphism of phenylaminobenzoic acids, five 3-methyl-2-(phenylamino)benzoic acids (MPABAs) were synthesized. A preliminary polymorph screen led to one form for compounds 1–4 and three forms and a solvate (5-I, 5-II, 5-III, and 5-S) for compound 5. This observation is similar to that of the structurally related compounds with no substitution on the benzoic acid ring. All crystal forms were characterized by single-crystal X-ray diffraction, powder X-ray diffraction (PXRD), and FT-IR spectroscopy. The polymorphs of compound 5 were investigated in detail experimentally and theoretically. A conformational scan provided insight into the origin of polymorphism of 5. NBO calculations confirmed the composition and nature of the sigma bond character of the two N–C bonds in 5. Hirshfeld analysis accounted for the major intermolecular interactions contributing to the overall stability of the crystals. It is clear that conformational flexibility alone doesn't lead to polymorphism, and substitution position and pattern are the real determinant of polymorphism in the investigated compounds.


 Please wait while we load your content...
Please wait while we load your content...