What roles do alkali metal ions play in the pathological crystallization of uric acid?†
Abstract
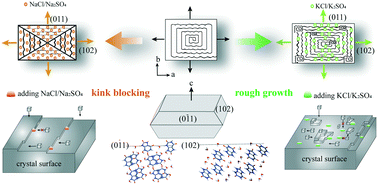
Uric acid (UA) is the end product of purine metabolism and is widely found in nature. Excess uric acid will result in the deposition of pathological uric acid crystals in the kidney. However, few studies have focused on the effects of components in the human body on the crystallization of uric acid. Herein, we found multiple effects of alkali metals (Na+, K+) on uric acid dihydrate (UAD) crystal growth, which is one of the main components of urinary stones. Optical microscopy (OM) and scanning electron microscopy (SEM) were employed to detect the crystal morphology, and long rod-like crystals and hierarchical crystals were found in the presence of Na+ and K+, respectively. Powder X-ray diffraction (PXRD) and micro-Raman spectroscopy verified that the crystal structure remained unchanged in the presence of ions. In addition, single-crystal growth experiments revealed that Na+ inhibited growth along [010] and [100], and K+ promoted growth along [100] but suppressed growth along [010]. The step growth of the UAD crystal was measured by atomic force microscopy (AFM), which indicated the inhibition of crystal growth by Na+ due to kink blocking, yet the promotion of crystal growth by K+ resulted from rough growth. Our studies showed that ions in the human body not only can maintain electrolyte balance but also can affect the crystallization of pathological crystals, which provides a suggestion for a rational diet for patients with hyperuricemia and urinary stones.


 Please wait while we load your content...
Please wait while we load your content...