Cationic complex directed thiostannate layers with excellent proton conduction and photocatalytic properties†
Abstract
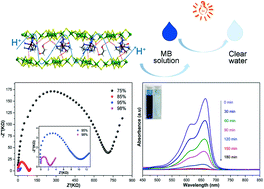
Three isostructural thiostannates SnS–M (M = Fe, Mn and Zn) have been fabricated using metal–amine complex cations as structure-directing agents. These thiostannates are composed of typical two-dimensional lamellar [Sn3S7]n2n− anionic structures with metal–amine complex cations located between the layers. Solid-state ultraviolet visible diffuse reflectance spectroscopy and Mott–Schottky plots reveal that the compounds possess n-type semiconducting behaviors with wide optical band gaps of 2.88, 2.96 and 3.02 eV, respectively. Abundant networks of hydrogen-bonds provide a high proton-conductivity value of 4.4 × 10−4 S cm−1 to SnS–Fe under 98% relative humidity (rh) at room temperature (rt, viz. 25 °C). In addition, SnS–Fe shows highly efficient photocatalytic degradation activity (of ∼100%) over organic pollutants.


 Please wait while we load your content...
Please wait while we load your content...