Supramolecular interaction of inositol phosphates with Cu(ii): comparative study of InsP6–InsP3†
Abstract
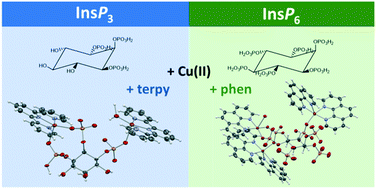
myo-inositol phosphates are an important group of biomolecules that are present in all eukaryotic cells. The most abundant member of this family in nature is InsP6 (H12L1), which interacts strongly with inorganic and organic cations. This interaction is essential for determining the possible functions of this biomolecule. A few crystal structures containing InsP6, and a divalent cation, have been reported showing a great versatility of this bioligand. Ins(1,2,3)P3 (H6L2) is another important member of the group, usually thought as a safe cellular iron ligand. No crystal structures showing the interaction of L2 with metal ions can be found in the literature. In this work we characterized by X-ray diffraction two polynuclear complexes, [Cu3(H6L1)(phen)5] (phen = 1,10-phenanthroline) (1) and [Cu2(H2L2)(H2O)(terpy)2] (terpy = 2,2′:6′,2′′-terpyridine) (2). The crystal structure of 2 furnishes, for the first time, a picture of the coordination ability of Ins(1,2,3)P3 against a divalent metal ion. In addition, a PDB survey was performed on all InsPs to frame the coordination modes derived from our small-molecule supramolecular approach within a more realistic biological context.


 Please wait while we load your content...
Please wait while we load your content...