Self-flux-grown Ba4Fe4ClO9.5−x crystals exhibiting structures with tunable modulation†
Abstract
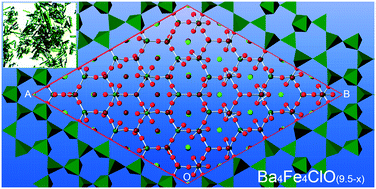
The synthesis and X-ray structural study of the new family of compounds Ba4Fe4ClO9.5−x with tunable structural modulation are reported. The framework of the structure has the Ba2Fe4O9.5−x composition, with open hexagonal channels extending along the c-axis. The channels are filled with linear [Ba–Cl–Ba] triplets. The oxygen stoichiometry and the oxidation state of iron both are controlled by the redox conditions during crystal preparation. The modulation of the crystal structure arises from the distribution of the oxygen atoms in the framework and iron coordination polyhedra are a combination of FeO4-tetrahedra, FeO5-bipyramids, and FeO6-octahedra. The structure modulation also originates from the ordered or disordered distribution of the [Ba–Cl–Ba] triplets filling the channels which is also affected by the conditions of the thermal treatment of the crystals. The structure investigation reveals a composition variation from Ba4Fe4ClO9.5 (x = 0), in which Fe exhibits a 3+ oxidation state, to Ba4Fe4ClO8 (x = 1.5) with the framework built exclusively of FeO4 tetrahedra.


 Please wait while we load your content...
Please wait while we load your content...