Constructing oxygen vacancy-enriched Fe2O3@NiO heterojunctions for highly efficient electrocatalytic alkaline water splitting†
Abstract
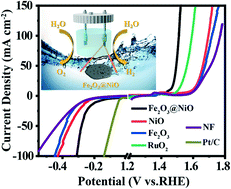
Electrolysis of water to produce high-purity hydrogen is a very promising method. The development of green, high-efficiency, long-lasting and low-cost dual function electrocatalysts for oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) is essential for electrocatalytic total water splitting. In this work, oxygen vacancy-enriched Fe2O3@NiO heterojunctions as bifunctional electrocatalysts are prepared through a facile one-step hydrothermal method followed by a calcination process. The synergistic effect of Fe2O3 and NiO, as well as the rich oxygen vacancies in Fe2O3, optimize their electronic structures, leading to an enhanced charge transfer rate and improved catalytic ability. Therefore, in both OER and HER processes, overpotentials needed for the Fe2O3@NiO catalyst to achieve the current density of 10 mA cm−2 under alkaline conditions are 224 mV and 187 mV, respectively. Furthermore, the catalyst showed excellent dynamic characteristics and durability. This research provides a new strategy for regulating the electronic structure of bifunctional catalysts by heterostructures and oxygen vacancies, thereby promoting the performance of total water splitting.


 Please wait while we load your content...
Please wait while we load your content...