Very close I⋯As and I⋯Sb interactions in trimethylpnictogen-pentafluoroiodobenzene cocrystals†
Abstract
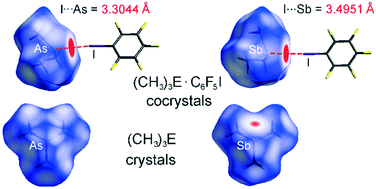
The cocrystals (CH3)3As·C6F5I (1) and (CH3)3Sb·C6F5I (2) were generated in situ from equimolar mixtures of their components. 1 and 2 show very close I⋯As and I⋯Sb directional intermolecular interactions. They are 0.5 and 0.7 Å shorter than the sums of van der Waals radii, respectively, and are the shortest C–I⋯As and C–I⋯Sb halogen bonds of this type found for experimentally characterized molecular (co)crystals. Comparisons of the packing motifs and contacts in 1 and 2 with those in (CH3)3As (3), (CH3)3Sb (4) and C6F5I (5) illustrate the occurrence and hierarchy of the specific interactions. The heteromolecular components in 1 and 2 are assembled by I⋯As, I⋯Sb and F⋯H interactions. There are no significant intermolecular As⋯As contacts in 3, but Sb⋯Sb interactions in 4. Molecules in 5 are mainly associated by I⋯F, F⋯F and F⋯C contacts. The intermolecular interactions observed in the (co)crystals correspond to the calculated electrostatic potentials.


 Please wait while we load your content...
Please wait while we load your content...