Facile synthesis of sulfonyl fluorides from sulfonic acids†
Abstract
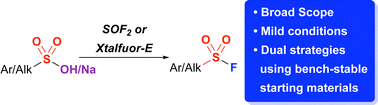
Herein, we demonstrate two complementary strategies for the syntheses of sulfonyl fluorides using sulfonic acids and their salts. One strategy involves the conversion of sulfonic acid sodium salts to sulfonyl fluorides using thionyl fluoride in 90–99% yields in one hour. Lessons learned from the mechanism of this reaction also have enabled a complementary deoxyfluorination of sulfonic acids using Xtalfluor-E® – a bench stable solid – allowing for the conversion of both aryl and alkyl sulfonic acids and salts to sulfonyl fluorides in 41–94% yields. Notably, using Xtalfluor-E® enabled milder conditions and the use of both sulfonic acids and their sodium salts.


 Please wait while we load your content...
Please wait while we load your content...