Chiral phosphoric acid-catalyzed dual-ring formation for enantioselective construction of N–N axially chiral 3,3′-bisquinazolinones†
Abstract
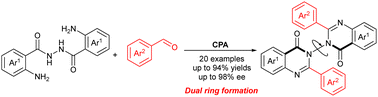
In contrast with the well-developed C–C and C–N axial chirality, research focusing on the catalytically asymmetric synthesis of N–N axially chiral compounds is still limited. As a privileged subunit of many antibiotics, the synthesis of N–N axially chiral 3,3′-bisquinazolinones has not been updated with atroposelective construction. Herein, we firstly report a chiral phosphoric acid-catalyzed dual-ring formation strategy leading to the aforementioned compounds with good chemical yields and enantioselectivities. Notably, metal-free reaction conditions are another advantage of this procedure.


 Please wait while we load your content...
Please wait while we load your content...