Total syntheses of wentilactones A and B, and related norditerpene dilactones†
Abstract
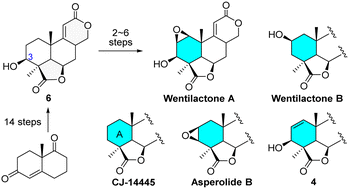
Wentilactones A and B, two representative tetranorditerpenoid dilactones with promising antitumor activities, are synthesized for the first time. The synthesis employs 3β-hydroxydilactone 6 as a common precursor, which is derived from (S)-Wieland–Miescher ketone, and leads easily to derivatives with various modifications on the A-ring, such as CJ-1445, asperolide B, and the unnamed natural congener 4.


 Please wait while we load your content...
Please wait while we load your content...