Concise enantioselective synthesis of non-proteinogenic α-aminoacids via an organocatalytic Mannich-type reaction†
Abstract
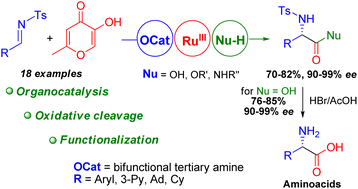
A bifunctional tertiary amine-squaramide-catalyzed enantioselective Mannich-type addition of available allomaltol to N-protected aldimines was developed. The resulted adducts were readily transformed into non-proteinogenic α-amino acids and their derivatives were not easily accessible by other methods via oxidative fragmentation, esterification, amidation and deprotection reactions. The absolute (S)-configuration of the thus obtained α-amino acids was established by comparison of the N-Ts-PheGly optical rotation sign with the reported data.


 Please wait while we load your content...
Please wait while we load your content...