Azobenzene-based unnatural amino acid scaffolds via a Pd(ii)-catalyzed C(sp3)–H arylation strategy†
Abstract
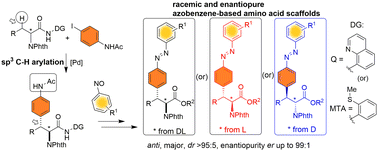
Azobenzene-based unnatural amino acid motifs were synthesized via Pd(II)-catalyzed diastereoselective C(sp3)–H arylation of amino acid carboxamides with iodoacetanilides and Mills azo coupling.


 Please wait while we load your content...
Please wait while we load your content...