Dynamic covalent polymers enabled by reversible isocyanate chemistry
Abstract
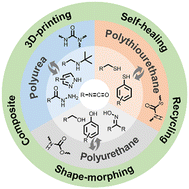
The design of responsive materials by introducing dynamic covalent bonds (DCBs), which can undergo reversible association and dissociation under certain conditions, is an appealing research field in recent years. Reversible isocyanate chemistry representatively consists of urethane, thiourethane, and urea bonds, all of which can reverse into their own starting chemicals (isocyanates and active hydrogen compounds) upon heating. In this article, we overview the mechanisms and experimental elements affecting the dynamic features of isocyanate-based bonds (IBs). With the knowledge of reversible isocyanate chemistry, the construction strategies of different dynamic covalent polymers including polyurethanes, polyureas, and polythiourethanes are discussed, in particular for dynamic polymer networks. The major applications of dynamic isocyanate-based polymers in recycling and self-healing materials, shape morphing polymers, 3D printing, and composites are outlined. The emergence of reversible isocyanate chemistry offers a highly effective platform to engineer thermally adaptable materials.


 Please wait while we load your content...
Please wait while we load your content...